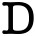thegavoice.com
prevention
HIV
Provident
FDA Approves First Injectable PrEP Medication
Reading now: 919
thegavoice.com
The U.S. Food and Drug Administration (FDA) has approved the first injectable treatment for preventing HIV. The new drug, Apretude, requires two shots one month apart followed by one shot every two months, offering a more convenient alternative to Truvada, a daily pill that is currently the most common form of HIV pre-exposure prophylaxis (PrEP).
According to a press release from the FDA, the first Apretude trial compared its results to Truvada’s among a sample of cisgender men and transgender women who have sex with men.
Participants using Apretude were 69 percent less likely to get HIV. In another trial, a sample of cisgender women taking Apretude were found to be 90 percent less likely to get HIV than those taking Truvada.
Read more on thegavoice.com
The website celebsbar.com is an aggregator of news from open sources. The source is indicated at the beginning and at the end of the announcement. You can send a complaint on the news if you find it unreliable.
 thegavoice.com
thegavoice.com